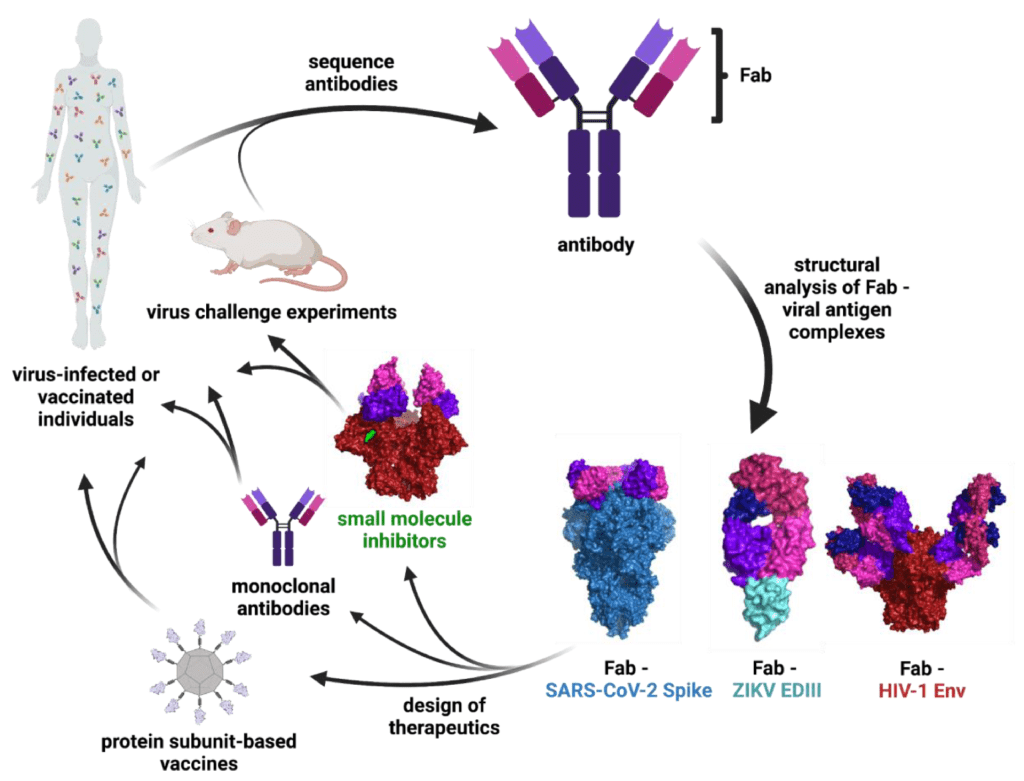
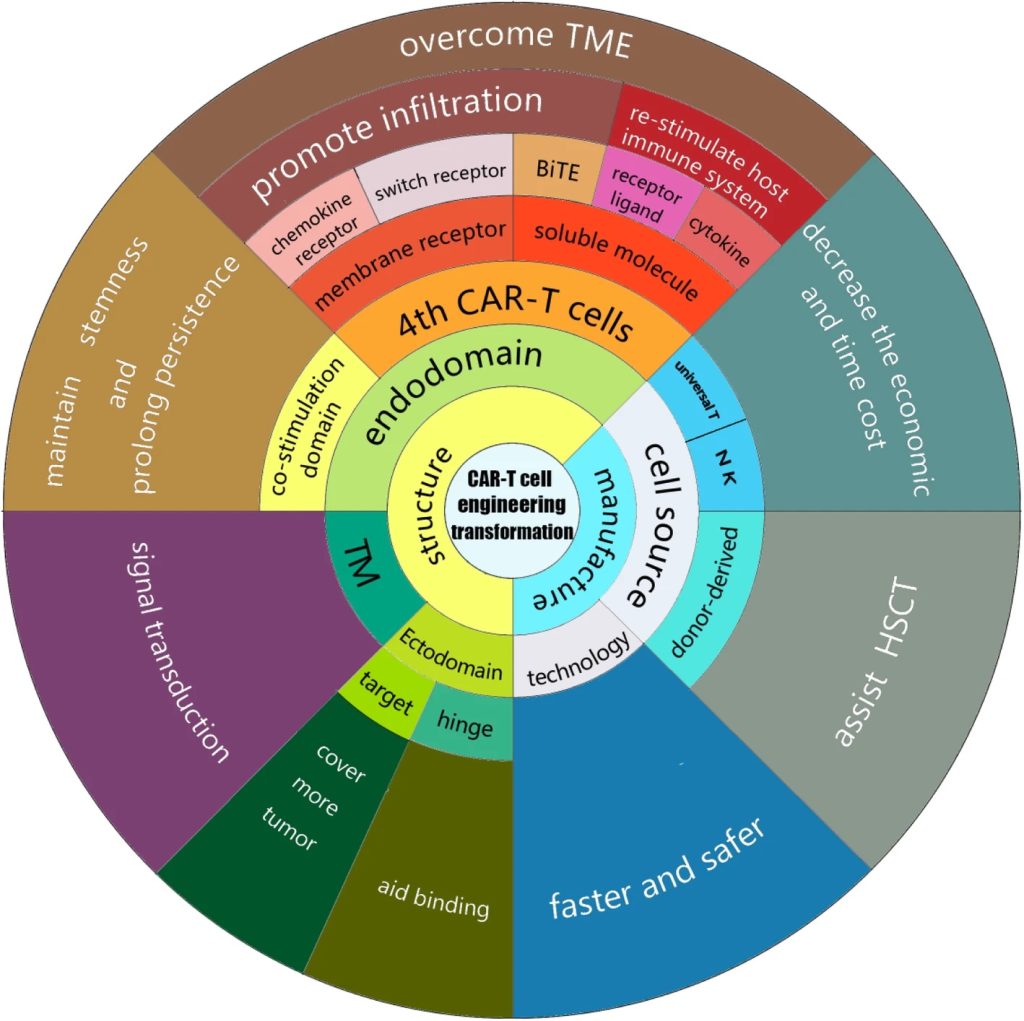
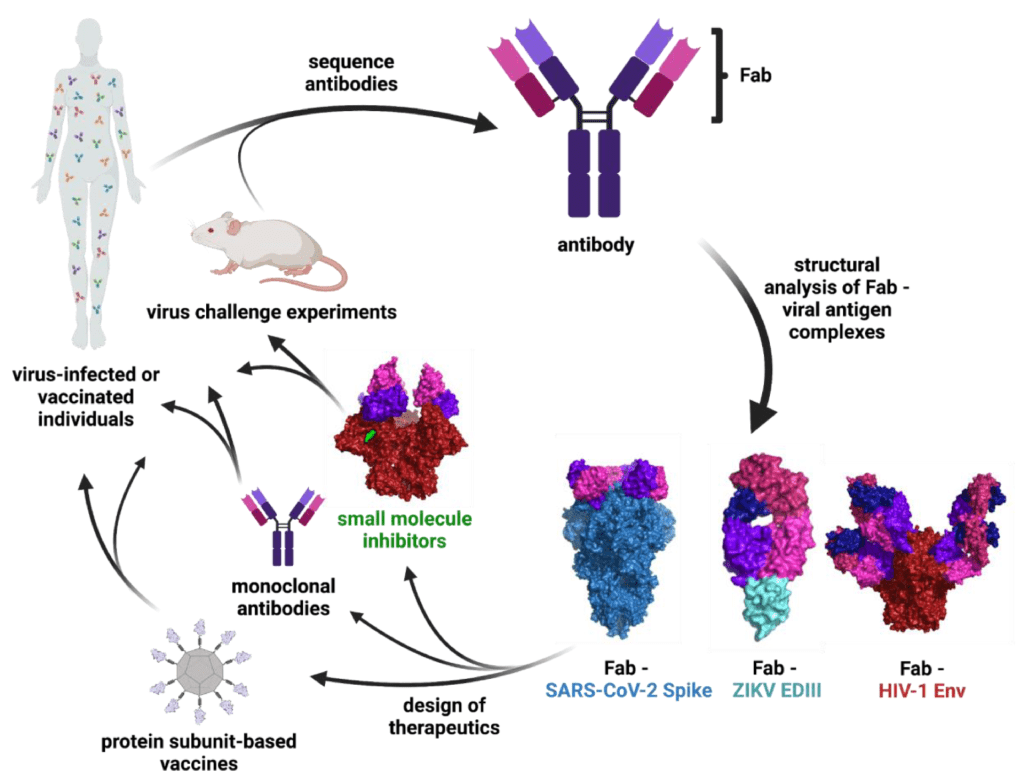
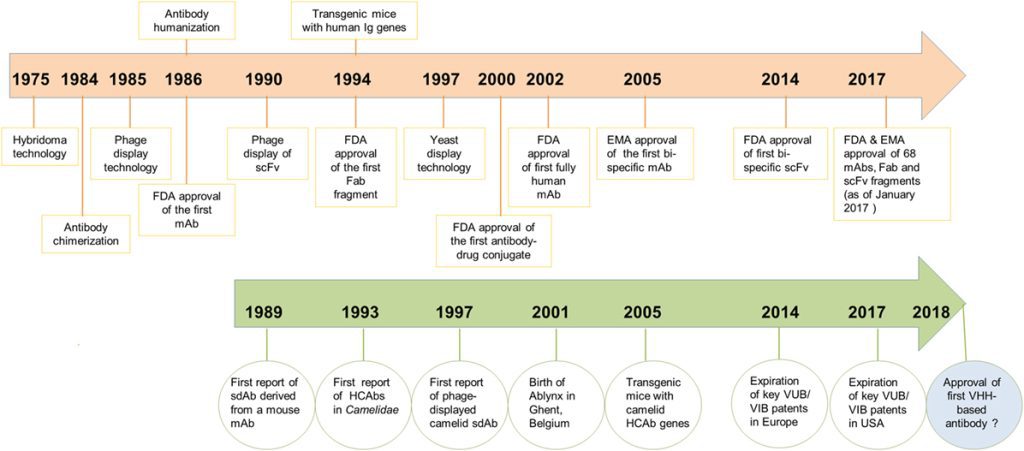
Unlocking the potential of IVDs: exploring regulatory updates
Jonathan Ripley, IVD and MD consulting services director at IMed Consultancy discusses the role of IVDs, regulatory perspectives for their adoption and increased market access. The EU has recently proposed an extension to the transitional provisions for certain IVD devices, postponing deadlines once again. Specifically, the proposal amends Article 110 IVDR and grants additional time to […]
Unlocking the potential of IVDs: exploring regulatory updates Read More »